Characteristics of Recombinant Protein COVID-19 Vaccine
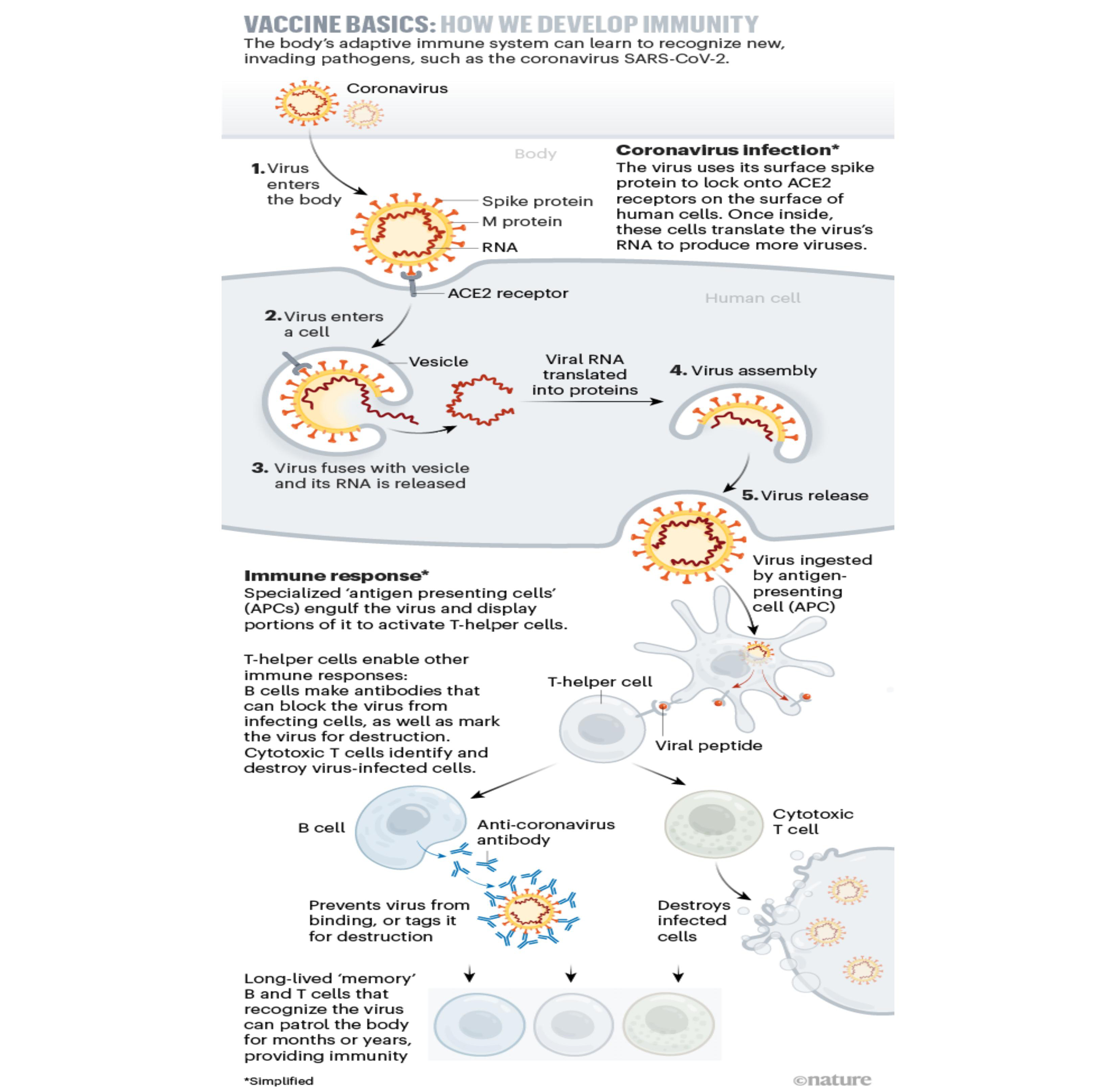
As the most effective and economical means to prevent and control the infection of the SARS-CoV-2, the R&D and application of the COVID-19 vaccine have always attracted much attention. The development of the COVID-19 vaccine has applied a variety of technical routes, namely inactivated vaccines, recombinant protein vaccines, adenovirus vector vaccines, attenuated influenza virus vector vaccines, and nucleic acid vaccines.What is a recombinant protein COVID-19 vaccine?For all we know, the SARS-CoV-2 mainly relies on the "Spikes" on its surface (called S protein in professional terms) to invade human cells and cause disease. In layman's terms, the SARS-CoV-2 is like a bad guy who usually uses its arms to do bad things. Just like the SARS-CoV-2 relies on the "Spikes" on its surface to invade human cells, speaking of which if we somehow bind the bad guy's arm, is it possible to stop it from doing bad things?
The design principle of COVID-19 vaccine
The same is true for the R&D principle of recombinant protein COVID-19 vaccine, thereof, if the vaccine developed by scientists precisely targets the S protein that the SARS-CoV relies on to invade human cells, and prompts the human body to produce antibodies that specifically bind to it, then when the human body is exposed to the SARS-CoV-2 again, it will produce a large amount of specific antibodies to block the combination of the SARS-CoV-2 and human cells, and play the protective role of the vaccine, which is equivalent to tying the arm of the bad guy, so that the bad guy can't do bad things! What are the advantages of recombinant protein COVID-19 vaccine?1. Making use of genetic engineering technology, recombinant protein vaccines can not only clone the protective antigen genes of pathogens, but also modify them in vitro, with good safety and immunogenicity [1] and remains effective against SARS-CoV-2 variant strains.SARS-CoV-2 variant strains are emerging continuously all over the world, and whether the SARS-CoV-2 variant strains can be prevented is also the focus of everyone's attention. The research findings show that the second-generation recombinant protein COVID-19 vaccine CoviccineTM -Recombinant COVID-19 vaccine (sf9 cells) developed by WestVac Biopharma has good safety and immunogenicity, and has high levels of neutralizing antibodies against SARS-CoV-2 variant strains such as Omicron and Delta, and has a long-lasting immune effect. Furthermore, a sequential third inoculation of CoviccineTM in the following of existing COVID-19 vaccines on the market significantly increased neutralizing antibody titers against multiple variant strains, fully reflecting the advantages and characteristics of the recombinant protein vaccine technical route. 2. There is no need to culture live viruses and worry about virus leakage, the production process is safe.The outstanding representative of recombinant protein vaccines is hepatitis B vaccine, which has 30 years of experience in production and use, while the up-rising stars include hepatitis E vaccine, cervical cancer vaccine, recombinant protein influenza vaccine [2], etc.The recombinant protein COVID-19 vaccine developed by a scientific research team led by Professor WEI Yuquan, Academician of the Chinese Academy of Sciences and director of the National Key Laboratory of Biotherapy, West China Hospital of Sichuan University, is produced by making use of insect cells (Sf9 cells) expression. The insect cell - baculovirus expression vector system is one of the four major expression systems (insect cells, bacteria, yeast, and mammalian cell expression systems), it has been used in influenza vaccines for many years, and it is also widely used in the field of bioengineering, which is an important engineering cell for the production of protein drugs, has relatively low requirements on the biosafety level of the production workshop, and there is no need to worry about the risk of virus leakage. Expression through insect cells not only has good protein expression quality, but also has high safety and is easy to produce on a large scale.3. The conventional storage temperature of recombinant protein vaccine is 2-8 ℃, which is easy to store and transport, and do not have high requirements on the cold chain, so it is easy to inoculate. Its good thermal stability makes it a very convenient vaccine for countries with underdeveloped cold chain systems. On February 19, 2022, with the approval of the Joint Prevention and Control Mechanism of the State Council, the National Health Commission of the People's Republic of China has begun to deploy sequential booster immunizations. According to the COVID-19 vaccine sequential booster immunization strategy, at this stage, the recombinant protein COVID-19 vaccine sequential booster immunization can be implemented in people aged 18 and over who have been vaccinated with the COVID-19 inactivated vaccine for 6 months and have not completed the homologous booster immunization. Research data shows that the booster immunization method of the inactivated COVID-19 vaccine + the recombinant protein COVID-19 vaccine has a good safety. Compared with the subjects with the inactivated COVID-19 vaccine homologous booster, the antibody titer is higher, in particular, the protective effect against variant strains such as Omicron is more obvious[3].Broadly speaking, the recombinant protein COVID-19 vaccine has a very balanced immune effect, safety and cold chain adaptability, and has the advantage of high production capacity, which is a edge tool for the global fight against the SARS-CoV-2. The boosted immunization method of inactivated COVID-19 vaccine + recombinant protein COVID-19 vaccine will also provide a safe and efficient solution to the pandemic of COVID-19 variant strains, further consolidate the effectiveness of COVID-19 vaccination, which plays an important role in building a national immunity barrier and effectively curbing the spread of the COVID-19 pandemic. References:1.GENG Shufan, WU Dan and YU Wenzhou: Research and Development Progress of COVID-19 Recombinant Protein Vaccine. 20202.ALI H. ELLEBEDY RA. Antiviral vaccines: challenges and advances [M]//The Vaccine Book. 2016. 3.Nawal AlKaabi, Yun Kai Yang, Jing Zhang:Safety and immunogenicity of a heterologous boost with a recombinant vaccine, NVSI-06-07, in the inactivated vaccine recipients from UAE: a phase 2 randomised, double-blinded, controlled clinical trial.2021
